The Steam Phase Diagram
The data provided in the above steam tables can also be expressed in a graphical form. Figure below, illustrates the relationship between the Enthalpy and Temperature of the various states of water and steam; this is known as a phase diagram.
As water is heated from 0°C to its saturation temperature, its condition follows the saturated water line until it has received all of its liquid enthalpy, hf, (A-B).
If further heat continues to be added, the water changes phase to a water/vapor mixture and continues to increase in enthalpy while remaining at saturation temperature, hfg, (B – C).
As the water/vapor mixture increases in dryness, its condition moves from the saturated liquid line to the saturated vapor line. Therefore at a point exactly halfway between these two states, the dryness fraction (Χ) is 0.5. Similarly, on the saturated steam line the steam is 100% dry.
Once it has received all of its enthalpy of evaporation, it reaches the saturated steam line. If it continues to be heated after this point, the pressure remains constant but the temperature of the steam will begin to rise as superheat is imparted.
The saturated water and saturated steam lines enclose a region in which a water/vapor mixture exists – wet steam. In the region to the left of the saturated water line only water exists, and in the region to the right of the saturated steam line only superheated steam exists.
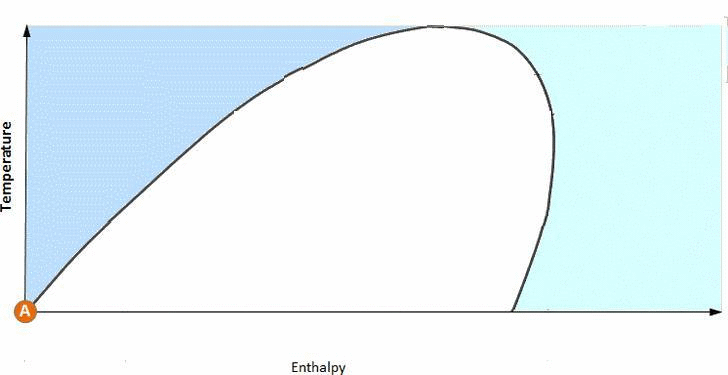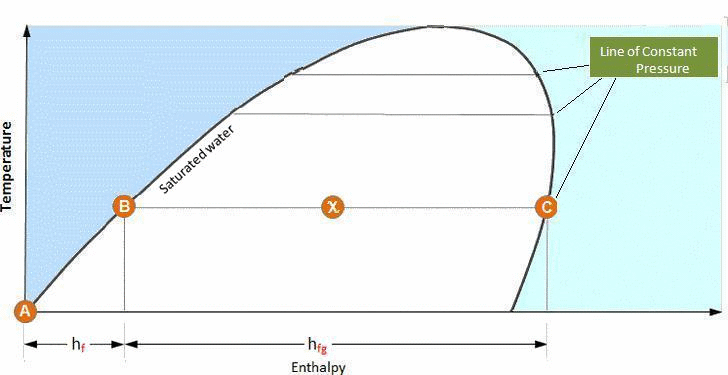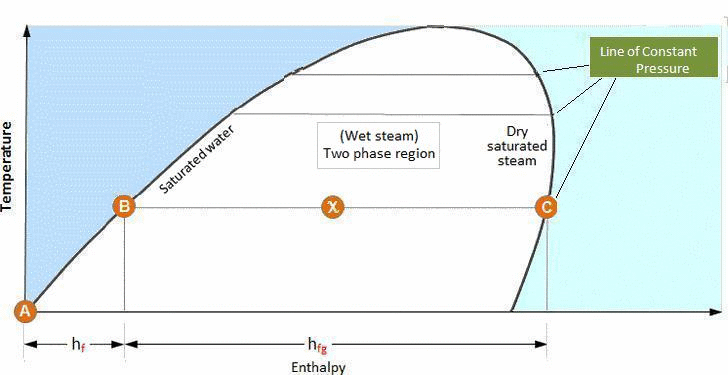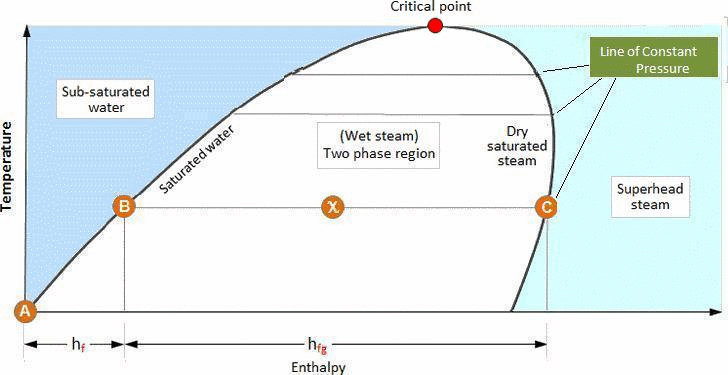
Critical Point :
The point at which the saturated water and saturated steam lines meet is known as the critical point. As the pressure increases towards the critical point the enthalpy of evaporation decreases, until it becomes zero at the critical point. This suggests that water changes directly into saturated steam at the critical point.
Above the critical point the steam may be considered as a gas. The gaseous state is the most diffuse state in which the molecules have an almost unrestricted motion, and the volume increases without limit as the pressure is reduced.
The critical point is the highest temperature at which water can exist. Any compression at constant temperature above the critical point will not produce a phase change.
Compression at constant temperature below the critical point however, will result in liquefaction of the vapor as it passes from the superheated region into the wet steam region.
The critical point occurs at 374.15°C and 221.2 bar-a for steam. Above this pressure the steam is termed supercritical and no well-defined boiling point applies.
Specific Volume of Steam : The volume per unit of mass in “Cubic Meter per Kg” / “Cubic Feet per Pound”.
Specific Gravity of Steam : The mass per unit volume in ‘Kg per Cubic Meter’ / ‘Pound per Cubic Feet’.
However, steam at atmospheric pressure is of a limited practical use. This is because it cannot be conveyed under its own pressure along a steam pipe to the point of use.
As per the relation between Pressure and Volume of steam, (volume is reduced as pressure is increased) steam is usually generated in the boiler at a pressure of at least 7 bar g. The generation of steam at higher pressures enables the steam distribution pipes to be kept to a reasonable size.
As the steam pressure increases, the density of steam will also increase. As the specific volume is inversely related to the density, the specific volume will therefore, decrease with increasing pressure.
Figure shows the relationship of specific volume to pressure. This highlights that the greatest change in specific volume occurs at lower pressures, whereas at the higher end of the pressure scale there is much less change in specific volume.
Dryness fraction :
Steam with a temperature equal to the boiling point at that pressure is known as dry saturated steam. However, to produce 100% dry steam in an industrial boiler is rarely possible, and the steam will usually contain droplets of water.
In practice, because of turbulence and splashing, as bubbles of steam break through the water surface, the steam space contains a mixture of water droplets and steam.
Steam produced in any shell-type boiler, where the heat is supplied only to the water and where the steam remains in contact with the water surface, may typically contain around 5% water by mass.
If the water content of the steam is 5% by mass, then the steam is said to be 95% dry and has a dryness fraction of 0.95.
The actual enthalpy of evaporation of wet steam is the product of the dryness fraction (Χ) and the specific enthalpy (hfg) from the steam tables. Wet steam will have lower usable heat energy than dry saturated steam.
Example :
Steam at a pressure of 7.0bar-g, having a dryness fraction of 0.95 will only contain 95% of the enthalpy of evaporation of dry saturated steam at 7.0bar-g. The following calculations use figures from steam tables :
100% dry saturated steam at 7.0bar-g will have total enthalpy = 660.8 kCal/kg.
Steam with 95% dryness fraction will have = 100% Liquid enthalpy 171kCal/kg + 95% Enthalpy of evaporation (489.8kCal/kg X 0.95) = 636.3kCal/kg
Actual Specific volume at 7.0barg and 95% dryness = 0.245 m3/kg X 0.95 = 0.233 m3/kg.

 Aerated Concrete Block Industry
Aerated Concrete Block Industry  Brewery Industry
Brewery Industry  Captive Cogen Industry
Captive Cogen Industry  Chemical Industry
Chemical Industry  Dairy Industry
Dairy Industry  Edible Oil Industry
Edible Oil Industry  Fertilizer Industry
Fertilizer Industry  Hotel Industry
Hotel Industry  Pharma Industry
Pharma Industry  Rice Industry
Rice Industry  Rubber Industry
Rubber Industry  Soap Industry
Soap Industry  Sugar Industry
Sugar Industry  Textile Industry
Textile Industry  Tyre Industry
Tyre Industry